About Event
The 6th Cell Therapy Analytical Development Summit was dedicated to enhancing analytical methods to accelerate product progression through IND and clinical trials, securing regulatory approval more efficiently.
Unlike other events including the CAR TCR Summit, this meeting focused on the critical role of analytical departments and quality control in reducing turnaround times and meeting regulatory standards.
We were joined by industry leaders and 40+ expert speakers from companies like Bristol Myers Squibb, Takeda, and Kite Pharma to explore advances in immunophenotype, cell-based, and molecular assays. Attendees discussed phase-appropriate development, assay matrices, and specifications.
They engaged in 8+ hours of networking, data-driven case studies, and discussions to tackle challenges from product characterization to scale-up. Attendees left with actionable insights to expedite their analytical package and product release.
This Was Your Chance To:
1
Learn to navigate analytical development guidelines and method validation with KSQ Therapeutics, focusing on controversial aspects and early regulatory engagement
2
Discover how liquid handling robots, scheduling software, and organs-on-a-chip improve assay workflows with insights from Prime Medicine, Arsenal Biosciences, and Vertex Pharmaceuticals
3
Explore analytical development challenges from early trials to post-approval with Bristol Myers Squibb and the University of Minnesota, and discuss adapting methodologies to meet evolving needs
4
Address immunotherapy characterization challenges such as antigen specificity and T-cell functionality with Verismo, IN8bio, and Century Therapeutics, focusing on autologous vs. allogeneic therapies
5
Redefine potency assays with TR1X Bio and Vertex Pharmaceuticals, developing functional assays for engineered immune system evasion while balancing robustness with clinical trial needs
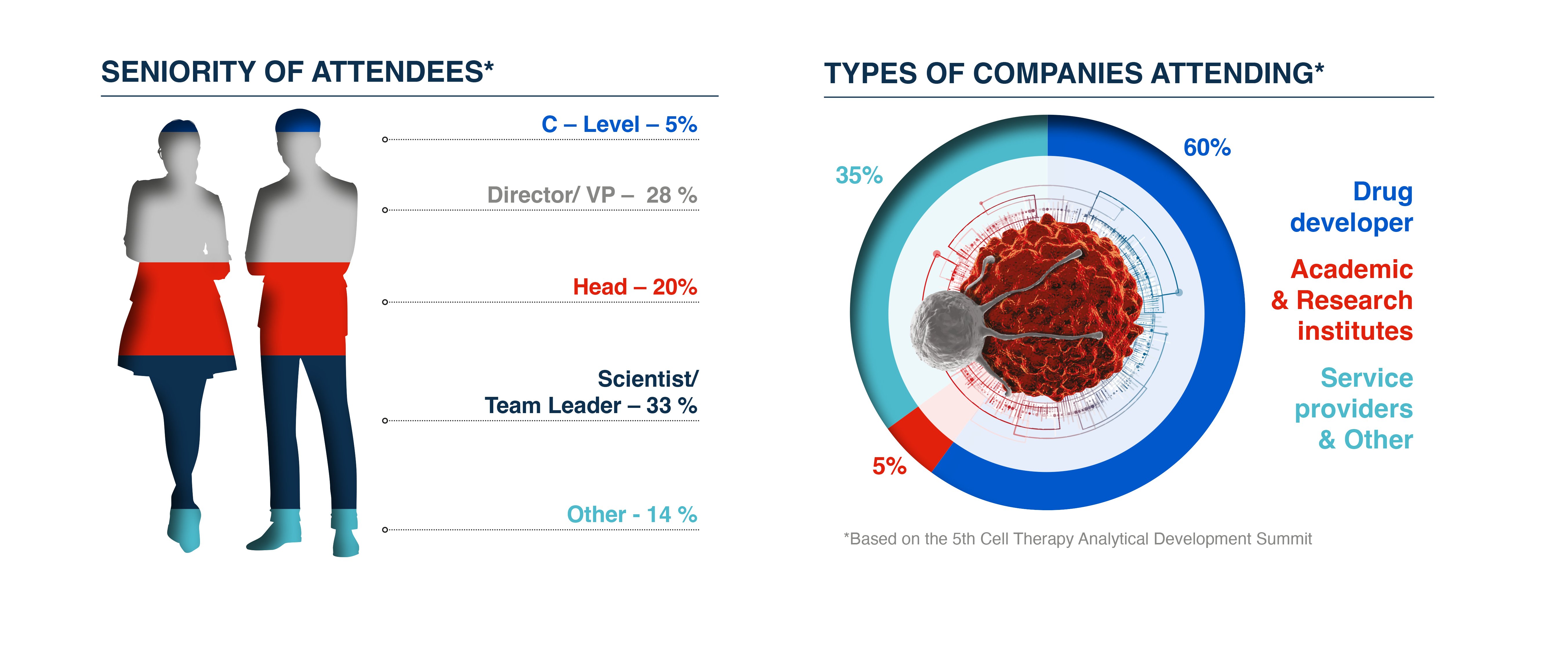
Who Would You Have Met?
This meeting united technical leads and decision-makers across cell therapy analytical development and quality control with expertise spanning across all assay types and technology focus working on early and late-stage cell therapy development to support learning across the analytical department.